Chemistry Journal of Moldova
Accepted papers
Field: Ecological chemistry
Type: Research paper
Type: Research paper
biosynthesis, silver, zinc, nanoparticle, photocatalysis.
https://doi.org/10.19261/cjm.2024.1113
Abstract (PDF)
Graphical Abstract: This study explores the green synthesis of silver and zinc nanoparticles using Laurel extract as a reducing agent. The biosynthesized nanoparticles were characterized for size, shape, and structure. Photocatalytic activities were evaluated for potential environmental applications. Results show promising prospects for sustainable nanoparticle synthesis and efficient photocatalytic degradation.
Field: Organic chemistry
Type: Research paper
Type: Research paper
multicomponent reaction, heteropolyacid, green chemistry approach, recyclable catalyst.
https://doi.org/10.19261/cjm.2024.1135
Abstract (PDF)
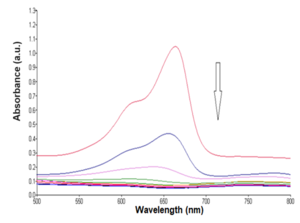
Graphical Abstract: This study presents an eco-friendly method for synthesizing 3,4-dihydropyrimidin-2(1H)-ones (DHPMs) through the Biginelli reaction. A novel Heteropolyacid-Clay (HPA-Clay) catalyst, formed by immobilizing H5PV2W10O40 on Montmorillonite KSF clay, displays enhanced stability and catalytic efficiency. Operating under solvent-free, one-pot conditions, the process delivers DHPMs with high yields and shortened reaction times. Catalyzed by 2 mol% HPA-Clay, it adheres to green chemistry principles, emphasizing cost-efficiency, environmental sustainability, and recyclability. The catalyst consistently performs over multiple cycles, showcasing promise for advancing Biginelli reactions.
Abstract (PDF)
Graphical Abstract: This study presents an eco-friendly method for synthesizing 3,4-dihydropyrimidin-2(1H)-ones (DHPMs) through the Biginelli reaction. A novel Heteropolyacid-Clay (HPA-Clay) catalyst, formed by immobilizing H5PV2W10O40 on Montmorillonite KSF clay, displays enhanced stability and catalytic efficiency. Operating under solvent-free, one-pot conditions, the process delivers DHPMs with high yields and shortened reaction times. Catalyzed by 2 mol% HPA-Clay, it adheres to green chemistry principles, emphasizing cost-efficiency, environmental sustainability, and recyclability. The catalyst consistently performs over multiple cycles, showcasing promise for advancing Biginelli reactions.

Field: Organic chemistry
Type: Review
Type: Review
acetophenone, vinyl-1,2,4-triazole, tetrazole-pyrazoline hybrid, Claisen-Schmidt condensation, chromenol.
https://doi.org/10.19261/cjm.2024.1139
Abstract (PDF)
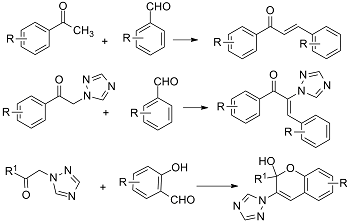
Graphical Abstract: The review is dedicated to the synthesis of 1,3-diaryl-2-propen-2-ones derived from aromatic methyl ketones. The article highlights advancements in the synthesis of chalcones and hybrid compounds based on chalcones containing 1,2,4-triazole, tetrazole-pyrazoline, and chromenol moieties. The biological activity of the synthesized compounds is comprehensively discussed.
Field: Natural product chemistry and synthesis
Type: Research paper
Type: Research paper
(+)-larixol, enolacetylation, dye-sensitized photooxidation, reduction, X-ray analysis.
https://doi.org/10.19261/cjm.2024.1156
Graphical Abstract: The main purpose of this research was the synthesis of highly functionalized derivatives of (+)-larixol by combination of classical and nonconventional method, like dye-sensitized photooxidation with preservation of outside chain. As a result, a series of four new cycle B derivatives of (+)-larixol were obtained. The structure of all synthesized compounds was fully confirmed by spectral method (IR, 1H and 13C NMR) and for compound containing endoperoxide functional group, additionally by single crystal X-ray diffraction analysis.