Chemistry Journal of Moldova
Inorganic and coordination chemistry
Author(s):
Field: Inorganic and coordination chemistry
Type: Research paper
Issue: 2013 Volume 8, no.1
Pages: 78-82
О. Ciobanica, P. Bourosh, О. Bologa, I. Bulhac, V. Lozan, V. Shofransky
Field: Inorganic and coordination chemistry
Type: Research paper
Issue: 2013 Volume 8, no.1
Pages: 78-82
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2013.08(1).09
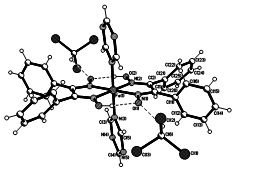
Graphical Abstract: The interaction of [Fe(DfgH)2Py2] (where DfgH=monodeprotonated diphenylglyioxime, Py-pyridune) and 1,3,5-triazine (Trz) in chloroform resulted in a new coordination compound with the composition [Fe(DfgH)2(Trz)2]·2CHCl3 (1). The crystal structure of 1, determined by single crystal X-ray diffraction, revealed that Fe(II) atom is coordinated by four oximic nitrogen atoms of two DfgH and two nitrogen atoms of two Trz ligands resulting in octahedral surrounding.
Downloads: 33
Author(s):
Field: Inorganic and coordination chemistry
Type: Research paper
Issue: 2012 Volume 7, no.2
Pages: 124-129
I. Voda, V. Druta, C. Indricean, I. Ciumacov, C. Turta
Field: Inorganic and coordination chemistry
Type: Research paper
Issue: 2012 Volume 7, no.2
Pages: 124-129
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2012.07(2).05
Graphical Abstract: By interaction of cobalt(II), nickel(II), or zinc(II) nitrate with 4,5- diphenylimidazole in methanol in solvotermal conditions the new derivative of imidazole (4,5-diphenyl-2-nitroimidazole) and three new coordinative compounds [M(4,5-Ph2ImNO2)2(CH3OH)2] have been synthesized and investigated. Metal ions have a distorted octahedral environment with N2O4. Coordination number of metal is six. Ligand is coordinated to metal ion by one oxygen atom of nitrogroup and one nitrogen atom of imidazole.
Downloads: 29
Author(s):
Field: Inorganic and coordination chemistry
Type: Review
Issue: 2010 Volume 5, no.1
Pages: 7-23
Vasile Lozan
Field: Inorganic and coordination chemistry
Type: Review
Issue: 2010 Volume 5, no.1
Pages: 7-23
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2010.05(1).01
Graphical Abstract: The coordination chemistry of dinickel macrocyclic
Downloads: 20
Author(s):
Field: Inorganic and coordination chemistry
Type: Review
Issue: 2010 Volume 5, no.1
Pages: 24-35
Vasile Lozan
Field: Inorganic and coordination chemistry
Type: Review
Issue: 2010 Volume 5, no.1
Pages: 24-35
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2010.05(1).02
Graphical Abstract: The steric protection offered by the macrobinucleating hexaazaditiophenolate
Downloads: 21
Author(s):
Field: Inorganic and coordination chemistry
Type: Research paper
Issue: 2010 Volume 5, no.1
Pages: 98-105
Gheorghe Nemtoi, Florica Ionica, Tudor Lupascu and Alexandru Cecal
Field: Inorganic and coordination chemistry
Type: Research paper
Issue: 2010 Volume 5, no.1
Pages: 98-105
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2010.05(1).10
Graphical Abstract: The dissolution of the iron from steel was observed by drawing the cyclic voltammetry (CV) for the systems consisting of the solution resulted when the alloy sample was immersed in HNO3, H2SO4, and HCl, aqueous solutions on platinum disk electrode (PtDE). The presence of some redox processes can be observed only in HNO3 which confirms the complexity of the mechanism of Fe dissolution in this acid. On the other hand, there were manufactured electrodes of steel samples taken into experiment achieving the corrosion characteristics in the media mentioned above.
Downloads: 22
Author(s):
Field: Inorganic and coordination chemistry
Type: Research paper
Issue: 2011 Volume 6, no.2
Pages: 70-72
Ştefan Manole, Maria Cocu
Field: Inorganic and coordination chemistry
Type: Research paper
Issue: 2011 Volume 6, no.2
Pages: 70-72
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2011.06(2).15
Graphical Abstract: We have researched the color properties of coordination compounds synthesized by us previously [1] (8-(1',2'-naphthyl)-1-
R3-methyl-6-thiomethyl-4,5,7-triazaocta-1,3,5,7-tetraenato-1,1'-diolato(-)O, O', N4, N7-M(II), where R=CH3, C6H5, M=Ni, Co, Cu), which can be used for coloring thermoplastic masses. They meet the requirements for use as a pigment for coloring thermoplastic masses.
Downloads: 32
Author(s):
Field: Inorganic and coordination chemistry
Type: Research paper
Issue: 2009 Volume 4, no.2
Pages: 68-71
S.B. Strashnova, O.V. Avramenko, M.N. Zhuk, O.V.Kovalchukova, P.V. Strashnov, B.E. Zaitsev
Field: Inorganic and coordination chemistry
Type: Research paper
Issue: 2009 Volume 4, no.2
Pages: 68-71
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2009.04(2).09
Graphical Abstract: The complexes of general formula MCl2∙L1-4∙nH2O (where L1 - N-(2,4,7-trinitrofluorenilidene-9)-p-dimethyl-aminoanilin, L2 - N-(2,4,5,7-tetranitrofluorenilidene-9)-p-dimethylaminoaniline, L3 - N-(2,4,7-trinitrofluorenilidene)-N-(p-dimethylaminophenyl)hydroxylamine, L4 - N-(2,4,5,7-tetranitrofluorenilidene-9)-N-(p-dimethylaminophenyl)-hydroxylamine; M=Cu, Co, Ni, Zn; n= 1-3 have been synthesized and investigated by different methods. Spectral criteria of co-ordination of the molecules L1 –L4 in electronic adsorption spectra were detected.
Downloads: 21
Author(s):
Field: Inorganic and coordination chemistry
Type: Research paper
Issue: 2009 Volume 4, no.2
Pages: 60-67
Silvia Melnic, Denis Prodius, Sergiu Shova, Helen Stoeckli-Evans, Yurii Simonov, Alexandr Feher, Maria Gdaniec, Constantin Turta
Field: Inorganic and coordination chemistry
Type: Research paper
Issue: 2009 Volume 4, no.2
Pages: 60-67
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2009.04(2).10
Graphical Abstract: Nine new complexes with the general formula {[Ln2Ba(α-Fur)8(H2O)4]}n, where Ln = Nd3+, Sm3+, Eu3+, Pr3+, Gd3+, Tb3+, Ho3+, Er3+ and La3+; α-Fur ≡ C4H3OCOO, were synthesized and characterized by IR spectra, magnetism, X-ray single crystal and powder diffractions.
Downloads: 26
Author(s):
Field: Inorganic and coordination chemistry
Type: Research paper
Issue: 2009 Volume 4, no.1
Pages: 90-96
Hideaki Ishida, Makoto Handa, Ichiro Hiromitsu, and Masahiro Mikuriya
Field: Inorganic and coordination chemistry
Type: Research paper
Issue: 2009 Volume 4, no.1
Pages: 90-96
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2009.04(1).06
Graphical Abstract: A chloro-bridged chain complex constructed from paddlewheel-type dinuclear ruthenium(II,III) carboxylate units, [Ru2{3,4,5-(C2H5O)3C6H2CO2}4Cl]n·1.2nC2H5OH (1·1.2nC2H5OH), was synthesized and characterized by elemental analysis and IR and UV-vis spectroscopies. The single-crystal X-ray analysis showed that the complex forms a zig-zag chain structure, in which the chloroligands bridge the dinuclear units at the axial positions with the Ru1–Cl–Ru2 angle of 120.38(7)°. A broad band around 1144 nm and a band at 475 nm were observed in the diffused reflectance spectra and ascribed to a δ→δ* and a π(RuO, Ru2)→π*(Ru2) transitions, respectively. Temperature dependence of magnetic susceptibility showed that the antiferromagnetic interaction between the dinuclear units is weak (zJ = −0.8 cm−1) with D value of 60 cm−1.
Downloads: 27
Author(s):
Field: Inorganic and coordination chemistry
Type: Research paper
Issue: 2008 Volume 3, no.2
Pages: 86-94
Sergiu Shova, Ana Lazarescu, Maria Gdaniec, Yurii Simonov, Constantin Turta
Field: Inorganic and coordination chemistry
Type: Research paper
Issue: 2008 Volume 3, no.2
Pages: 86-94
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2008.03(2).04
Graphical Abstract: Two novel μ3-oxo-centered carboxylate-bridged triiron complexes [Fe3O(BrCH2COO)6(H2O)3] NO3·2.63H2O (1) and [Fe3O(CH2BrCOO)1.5(CH2ClCOO)4.5(H2O)3]Br0.75Cl0.25 5H2O (2) were synthesized and their structures were characterized by X-ray crystallography. The opportunity of mixed-ligand complex formation in iron(III)-bromoacetic acid system was shown. The first co-ordination sphere of the iron atom in compound 2 includes two different carboxylate anions, CH2BrCOO- and CH2ClCOO- in the capacity of syn-syn- bidentate-bridged ligands, while Br- and Cl- anions being in the ratio 1:1, formulate the external sphere of the complex. The IR spectra, thermic analysis and magnetic properties of complexes were studied.
Downloads: 28






