Chemistry Journal of Moldova
Natural product chemistry and synthesis
Author(s):
Field: Natural product chemistry and synthesis
Type: Review
Issue: 2012 Volume 7, no.2
Pages: 46-56
V. Kulcitki
Field: Natural product chemistry and synthesis
Type: Review
Issue: 2012 Volume 7, no.2
Pages: 46-56
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2012.07(2).14
Graphical Abstract: The current paper represents an outline of the selected contributions to the biomimetic procedures and approaches for the synthesis of terpenes with complex structure and diverse functionalisation pattern. These include homologation strategies, cyclisations, rearrangements, as well as biomimetic remote functionalisations.
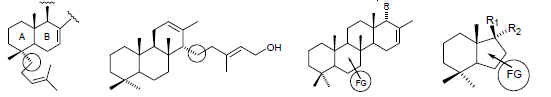
Downloads: 38
Author(s):
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2010 Volume 5, no.2
Pages: 54-58
Alexandra Marchenko, Pavel Kintia, Bożena Wyrzykiewicz
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2010 Volume 5, no.2
Pages: 54-58
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2010.05(2).07
Graphical Abstract: The paper reports on the structural elucidation of the four steroidal glycosides, where two are new, isolated from Veronica chamaedrys L. plants for the first time on the basis of extensive spectral analysis, including 2D NMR spectral data and chemical evidences.
Downloads: 23
Author(s):
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2010 Volume 5, no.2
Pages: 59-67
N. Secara, Gh. Duca, L. Vlad, F. Macaev
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2010 Volume 5, no.2
Pages: 59-67
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2010.05(2).08
Graphical Abstract: The paper describes the syntheses of new derivatives of dihydroxyfumaric acid and investigations of their antioxidant activities using the DPPH method.
Downloads: 35
Author(s):
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2010 Volume 5, no.1
Pages: 106-108
Marina Grinco, Olga Chetraru, Veaceslav Kulciţki, Alic Barba, Alexandr Boico, Pavel F. Vlad and Nicon Ungur
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2010 Volume 5, no.1
Pages: 106-108
Full Text (PDF): Download
Author(s):
Field: Natural product chemistry and synthesis
Type: Short communication
Issue: 2010 Volume 5, no.1
Pages: 118-120
Veaceslav Kulciţki, Marina Cara, Andrea Bourdelais, Tomas Schuster and Daniel Baden
Field: Natural product chemistry and synthesis
Type: Short communication
Issue: 2010 Volume 5, no.1
Pages: 118-120
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2010.05(1).13
Graphical Abstract: Conjugate 1,4-addition of N-bromosuccinimide (NBS) to a diene system, possessing a suitable oxygen functionality, leads to functionalized tetrahydrofuran derivatives, which can be further derivatized into different synthetic targets.
Downloads: 18
Author(s):
Field: Natural product chemistry and synthesis
Type: Short communication
Issue: 2010 Volume 5, no.1
Pages: 121-123
Olga Chetraru, Marina Grinco, Veaceslav Kulciţki, Pavel F. Vlad and Nicon Ungur
Field: Natural product chemistry and synthesis
Type: Short communication
Issue: 2010 Volume 5, no.1
Pages: 121-123
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2010.05(1).14
Graphical Abstract: The direct, one step conversion of ent-kaurenoic acid 3 into atisanic and beyeranic acids 1 and 2 was performed under the action of fluorosulfonic acid.
Downloads: 17
Author(s):
Field: Natural product chemistry and synthesis
Type: Short communication
Issue: 2011 Volume 6, no.2
Pages: 7-8
M. Gavagnin, V. Kulcitki
Field: Natural product chemistry and synthesis
Type: Short communication
Issue: 2011 Volume 6, no.2
Pages: 7-8
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2011.06(2).01
Moldovan chemists hosted the Italian partners in Chisinau and organized in premiere for the last 20 years an international seminar devoted to the chemistry of natural products. The seminar’s motto: New Frontiers in the Chemistry of Natural Products has addressed numerous students and researchers acting in the fields of chemistry, biology, pharmacy, genetics, biophysics as well as specialists from the R&D sector of companies with chemico-pharmaceutical profile. Following multiple solicitations coming from seminar audience, made us provide in the current issue of the “Chemistry Journal of Moldova. General, Industrial and Ecological Chemistry” a condensed abstract of the seminar presentations. We do really hope that our joint event will give further impetus to our collaboration and will also motivate other researchers from Moldova to develop partnerships with European colleagues.
Downloads: 16
Author(s):
Field: Natural product chemistry and synthesis
Type: Invited paper
Issue: 2011 Volume 6, no.2
Pages: 9-12
M. L. Ciavatta
Field: Natural product chemistry and synthesis
Type: Invited paper
Issue: 2011 Volume 6, no.2
Pages: 9-12
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2011.06(2).02
Graphical Abstract: The topic of the seminar held in the Institute of Chemistry, Academy of Sciences of Moldova on 30th September in the frame of the joint Moldo-Italian seminar “New frontiers in natural product chemistry”, concerned the use of NMR techniques in the elucidation of natural products. Step by step, two marine compounds (Fulvyne C and Tritoniopsin A) belonging to different chemical classes have been analyzed, by using suitable NMR experiments. This powerful technique allowed the elucidation of compounds as fulvynes, long chain polyacetylenes with the same functional groups but differently located in the chain, as well as tritoniopsins, cyclic diterpenes with a new skeleton, providing further information on their relative and absolute stereochemistry.
Downloads: 22
Author(s):
Field: Natural product chemistry and synthesis
Type: Short communication
Issue: 2011 Volume 6, no.2
Pages: 13-15
A. Ciocarlan, P. F. Vlad, M. Coltsa, C. Edu, A. Biriiac, A. Nicolescu and C. Deleanu
Field: Natural product chemistry and synthesis
Type: Short communication
Issue: 2011 Volume 6, no.2
Pages: 13-15
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2011.06(2).03
Graphical Abstract: The current communication represents an extended abstract of the presentation delivered on the joint Moldo-Italian seminar “New frontiers in natural product chemistry”, held in the Institute of Chemistry, Academy of Sciences of Moldova on 30 September. An overview of the synthetic methods oriented to the synthesis of C6 and C7 functionalized euryfurans is provided.
Downloads: 34
Author(s):
Field: Natural product chemistry and synthesis
Type: Short communication
Issue: 2011 Volume 6, no.2
Pages: 16-18
T. Cotelea
Field: Natural product chemistry and synthesis
Type: Short communication
Issue: 2011 Volume 6, no.2
Pages: 16-18
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2011.06(2).04
Graphical Abstract: The current communication includes a general overview of the scientific interest and medicianl chemistry aspects of Cannabis compounds. It relates to metabolism, pharmacological action and phisico-chemical analysis of these compounds, as well as of some isomers differing in spatial arrangement of functional groups.
Downloads: 25






