Chemistry Journal of Moldova
Natural product chemistry and synthesis
Author(s):
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2021 Volume 16, no.1
Pages: 99-104
Olga Morarescu, Marionela Traistari, Alic Barba, Gheorghe Duca, Nicon Ungur, Veaceslav Kulcițki
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2021 Volume 16, no.1
Pages: 99-104
Full Text (PDF): Download
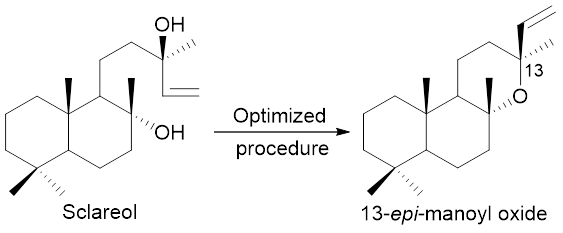
Graphical Abstract: The selective one-step synthesis of 13-epi-manoyl oxide is reported based on a low-temperature superacidic cyclization of sclareol. The reaction conditions have been finely tuned in order to achieve a 9:1 ratio between epimeric oxides in favor of the desired 13-epi-oxide.
Downloads: 136
Author(s):
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2020 Volume 15, no.2
Pages: 78-87
Brahim Ben Aicha, Rachid Rouabhi, Salim Gasmi, Chawki Bensouici, Hichem Mohammedi, Imad Mennai
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2020 Volume 15, no.2
Pages: 78-87
Full Text (PDF): Download
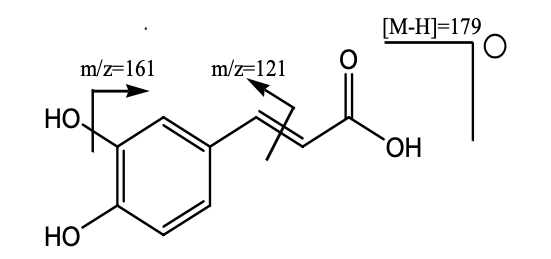
Graphical Abstract: The present work focuses on evaluation of the chemical composition and antioxidant activity of the hydro-methanolic extract of Melissa officinalis from Algeria. The liquid chromatography-mass spectrometry analysis allowed the identification of six compounds: caffeic acid, caftaric acid, hydroxyjasmonic acid glucoside, caftaric acid glucoside, rosmarinic acid and sagerinic acid. The in-vitro antioxidant activity of the hydro-methanolic extract was evaluated by using four different methods including: radical scavenging assay (DPPH), scavenging activity (ABTS), cupric reducing antioxidant capacity, and ferric reducing power assay. The extract exhibited a relatively strong antioxidant activity compared to the synthetic antioxidants. The highest radical scavenging activity was registered using DPPH and ABTS methods, IC50= 20.53±2.64 μg/mL and 22.50±0.67 μg/mL, respectively. These results suggest that Melissa officinalis L. could be considered a potential source of natural antioxidants with potential interest in the agrochemical and pharmaceutical industries.

Downloads: 232
Author(s):
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2020 Volume 15, no.2
Pages: 69-77
Alexandru Ciocarlan, Lidia Lungu, Svetlana Blaja, Ion Dragalin, Aculina Aricu
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2020 Volume 15, no.2
Pages: 69-77
Full Text (PDF): Download
Abstract (PDF)
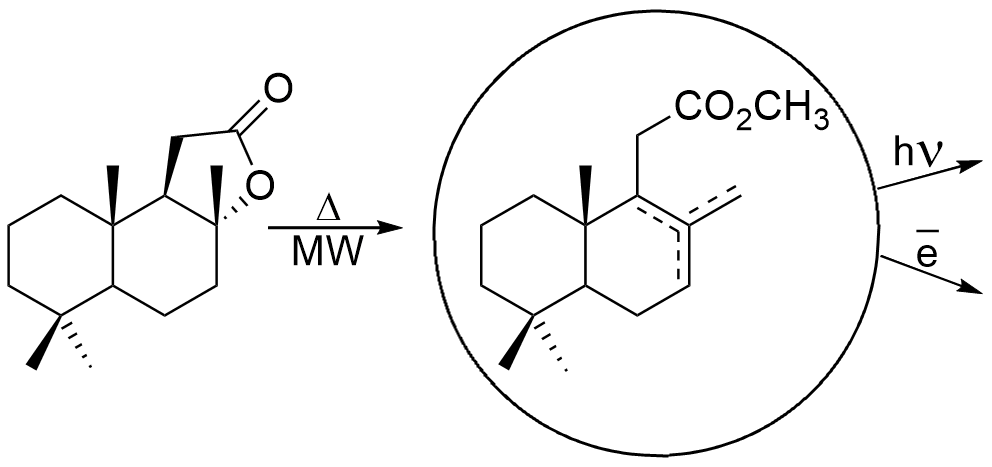
Graphical Abstract: The present paper reports the results of microwave irradiation assisted method for the preparation of bicyclohomofarnesenic methyl esters versus classical Stoll and Hinder method. Moreover, the chemical transformations of bicyclohomofarnesenic methyl esters via anodic electrooxidation and dye-sensitized photooxidation were performed. A new method for the preparation of methyl 7-oxo-13,14,15,16-tetranorlabd-6,8(8)-dien-12-oate and the mechanism of electrochemical products formation are presented. The structure of all synthesized compounds was fully confirmed by spectral methods.
Downloads: 187
Author(s):
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2019 Volume 14, no.2
Pages: 79-89
Hichem Mohammedi, Samira Idjeri-Mecherara, Fouad Menaceur, Aicha Hassani
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2019 Volume 14, no.2
Pages: 79-89
Full Text (PDF): Download
Abstract (PDF)
Graphical Abstract: This paper focuses on the study of the effect of extraction solvent choice on phenolic compounds contents and antioxidant activity of Bassia muricata. In this study, five different solvents namely: water, acetone, ethanol, methanol and hexane, and three extraction techniques were used to extract phenolic compounds: microwave-assisted extraction, Soxhlet and maceration. Total phenolics, total flavonoids and condensed tannins contents were determined. The results showed that different solvents with different polarity had a major effect on polyphenolic contents and antioxidant activity. Microwave-assisted extraction was the best suited for the extraction of antioxidant molecules when compared to Soxhlet and maceration.
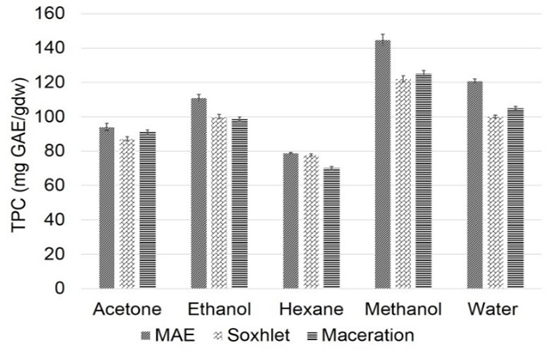
Graphical Abstract: This paper focuses on the study of the effect of extraction solvent choice on phenolic compounds contents and antioxidant activity of Bassia muricata. In this study, five different solvents namely: water, acetone, ethanol, methanol and hexane, and three extraction techniques were used to extract phenolic compounds: microwave-assisted extraction, Soxhlet and maceration. Total phenolics, total flavonoids and condensed tannins contents were determined. The results showed that different solvents with different polarity had a major effect on polyphenolic contents and antioxidant activity. Microwave-assisted extraction was the best suited for the extraction of antioxidant molecules when compared to Soxhlet and maceration.

Downloads: 209
Author(s):
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2019 Volume 14, no.2
Pages: 72-78
Svetlana Blaja
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2019 Volume 14, no.2
Pages: 72-78
Full Text (PDF): Download
Abstract (PDF)
Graphical Abstract: TThe present paper reports the synthesis of new hybrid terpeno-heterocyclic compounds belonging to di- and tri-norlabdane series. Starting from natural labdane diterpenoide (-)-sclareol, via its intermediates 8α-hydroxy-15,16-dinorlabd-13-one and sclareolide, two di-norlabdane and three tri-norlabdane, previously unreported compounds possessing 2-amino-1,3-thiazole structural units were obtained in three and four steps, respectively, with acceptable to good overall yields. The structures of newly obtained compounds were confirmed by means of spectral IR, 1H and 13C NMR analyses.
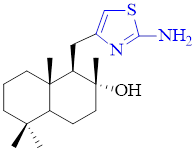
Graphical Abstract: TThe present paper reports the synthesis of new hybrid terpeno-heterocyclic compounds belonging to di- and tri-norlabdane series. Starting from natural labdane diterpenoide (-)-sclareol, via its intermediates 8α-hydroxy-15,16-dinorlabd-13-one and sclareolide, two di-norlabdane and three tri-norlabdane, previously unreported compounds possessing 2-amino-1,3-thiazole structural units were obtained in three and four steps, respectively, with acceptable to good overall yields. The structures of newly obtained compounds were confirmed by means of spectral IR, 1H and 13C NMR analyses.

Downloads: 163
Author(s):
Field: Natural product chemistry and synthesis
Type: Invited paper
Issue: 2019 Volume 14, no.2
Pages: 9-31
Margherita Gavagnin, Marianna Carbone, Maria Letizia Ciavatta, Ernesto Mollo
Field: Natural product chemistry and synthesis
Type: Invited paper
Issue: 2019 Volume 14, no.2
Pages: 9-31
Full Text (PDF): Download
Abstract (PDF)
Graphical Abstract: Heterobranchs are a fascinating group of marine mollusks that are recognized as an important source of bioactive natural products. Often, these molecules, which are either selected from the diet or de novo biosynthesized by the mollusks, play a fundamental role for their survival being utilized as defensive chemicals against predators. A summary of the studies carried out, in the last decade, on heterobranchs is presented here. A number of new compounds exhibiting different molecular architectures were chemically characterized.
Graphical Abstract: Heterobranchs are a fascinating group of marine mollusks that are recognized as an important source of bioactive natural products. Often, these molecules, which are either selected from the diet or de novo biosynthesized by the mollusks, play a fundamental role for their survival being utilized as defensive chemicals against predators. A summary of the studies carried out, in the last decade, on heterobranchs is presented here. A number of new compounds exhibiting different molecular architectures were chemically characterized.

Downloads: 99
Author(s):
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2018 Volume 13, no.2
Pages: 63-68
Alexandru Ciocarlan, Ion Dragalin, Aculina Aricu, Lucian Lupascu, Nina Ciocarlan, Violeta Popescu
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2018 Volume 13, no.2
Pages: 63-68
Full Text (PDF): Download
Graphical Abstract: The chemical composition of industrially obtained Levisticum officinale W.D.J. Koch (lovage) essential oil of Moldovan origin was analysed by means of chromatographic (GC-MS) and spectral (IR, 1H and 13C NMR) methods. The obtained results show that the main components of L. officinale essential oil are monoterpenic hydrocarbons which make up to 53.50% of the total number of components. L. officinale essential oil is also characterized by a high content of oxygenated monoterpenes (alcohols, cetones and esters), which reaches up to 33.60%. For the first time the presence of 6-butyl-cyclohepta-1,4-diene (0.56%) and 7-formyl-4-methyl-cumarine (0.15%) in lovage essential oil is reported. Antibacterial and antifungal activities of mentioned oil were evaluated in vitro on five strains of microorganisms. It was found that lovage volatile oil (L .officinale) exhibits high antibacterial and antifungal properties in the range of concentrations 0.015-0.030%.

Downloads: 234
Author(s):
Field: Natural product chemistry and synthesis
Type: Review
Issue: 2018 Volume 13, no.2
Pages: 8-23
Benalia Yabrir
Field: Natural product chemistry and synthesis
Type: Review
Issue: 2018 Volume 13, no.2
Pages: 8-23
Full Text (PDF): Download
Graphical Abstract: This paper reviews information on essential oil of Marrubium species (except M. vulgare) described until now regarding extraction, chemical composition and biological activities. Marrubium essential oils, although quantitatively poor, are rich in chemical composition. This composition consists especially of sesquiterpenoids and a little amount of monoterpenes. It varies from one species to another, sometimes within same species. Marrubium essential oils exhibit antioxidant and antimicrobial activities. However, because the lack of literature concerning essential oil of these species, further studies are necessary, particularly regarding their activities.

Downloads: 169
Author(s):
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2018 Volume 13, no.1
Pages: 63-68
Alexandru Ciocarlan, Ion Dragalin, Aculina Aricu, Nina Ciocarlan, Cristina Stavarache, Mariana Deleanu
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2018 Volume 13, no.1
Pages: 63-68
Full Text (PDF): Download
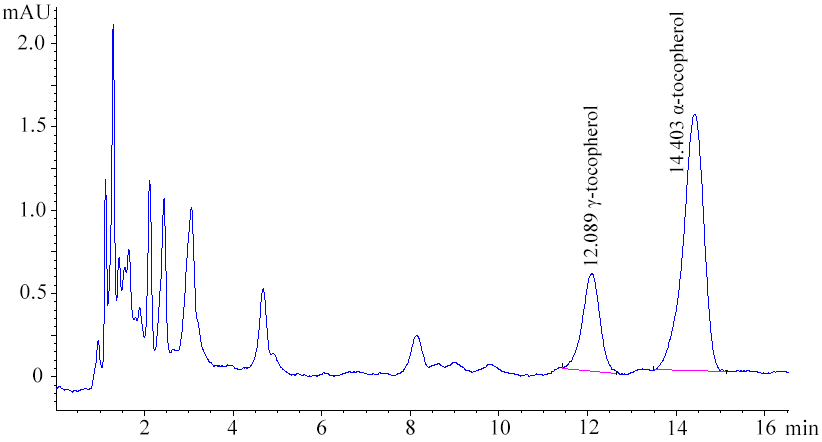
Graphical Abstract: The present paper describes biochemical (fatty oil) composition of Silybummarianum (L.) Gaertn. of Moldovan origin. The oil content of the seeds was approximately 25%. Linoleic acid (C18:2), an essential polyunsaturated fatty acid, is the most abundant (48.88%), followed by monounsaturated oleic acid (C18:1, 31.94%) and saturated palmitic acid (C16:0, 7.61%). The RP-HPLC analysis of tocopherols composition, showed as main components α-tocopherol (23.45 mg/100g) and γ–tocopherol (5.60 mg/100g). Based on the obtained results, it was shown that the extracted oil from milk thistle seeds is rich in essential fatty acids (about 50%) and tocopherols (29.09 mg/100g) and it can be used in food preparation.

Downloads: 188
Author(s):
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2017 Volume 12, no.2
Pages: 50-57
Nassiba Fekhar, Hocine Boutoumi, Mohamed Krea, Saâd Moulay, Drioueche Asma, Zoubir Benmaamar
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2017 Volume 12, no.2
Pages: 50-57
Full Text (PDF): Download
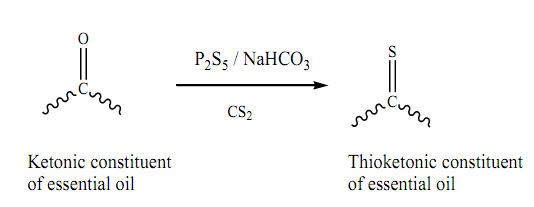
Graphical Abstract: Essential oils were extracted from Artemisia herba-alba L. and Ruta montana L. by means of steam distillation and thionated with a reagent combination of phosphorus pentasulfide and sodium bicarbonate. Both parent essential oils and their modified ones were screened for their biological and insecticidal activities. The results showed that essential oils were composed mainly of ketones; essential oils from Artemisia herba-alba L. and those from Ruta montana L. consisted of bicyclic monoterpenes and acyclic aliphatic ketones (thujone, camphor and 2-undecanone), respectively. The antimicrobial activity of essential oils was substantially improved upon thionation (from 10 to 34 mm and from 11 to 32 mm). The insecticidal effect of the thionated essential oil from Ruta montana L. was observed to be very significant, but that of the essential oil from Artemisia herba-alba L. was observed to decrease (from 100% to 70% after 24 hrs. The extracted essential oils as well as their thionated forms were characterized by GC-MS, FT-IR, and UV-visible.

Downloads: 183






