Chemistry Journal of Moldova
COMPOUNDS REMOVED FROM THE CONDENSATION REACTION BETWEEN 2-ACETYLPYRIDINE AND 2-FORMYLPYRIDINE. SYNTHESIS, CRYSTAL STRUCTURE AND BIOLOGICAL EVALUATION
Author(s):
Field: Organic chemistry
Type: Research paper
Issue: 2020 Volume 15, no.2
Pages: 88-98
Roman Rusnac, Maria Botnaru, Nicanor Barba, Peter Petrenko, Yurii Chumakov, Aurelian Gulea
Field: Organic chemistry
Type: Research paper
Issue: 2020 Volume 15, no.2
Pages: 88-98
1,3-bis(pyridin-2-yl)prop-2-en-1-one, Claisen-Schmidt condensation, intramolecular aldol condensation, Michael addition, substituted cyclohexanol.
Full Text (PDF): Download
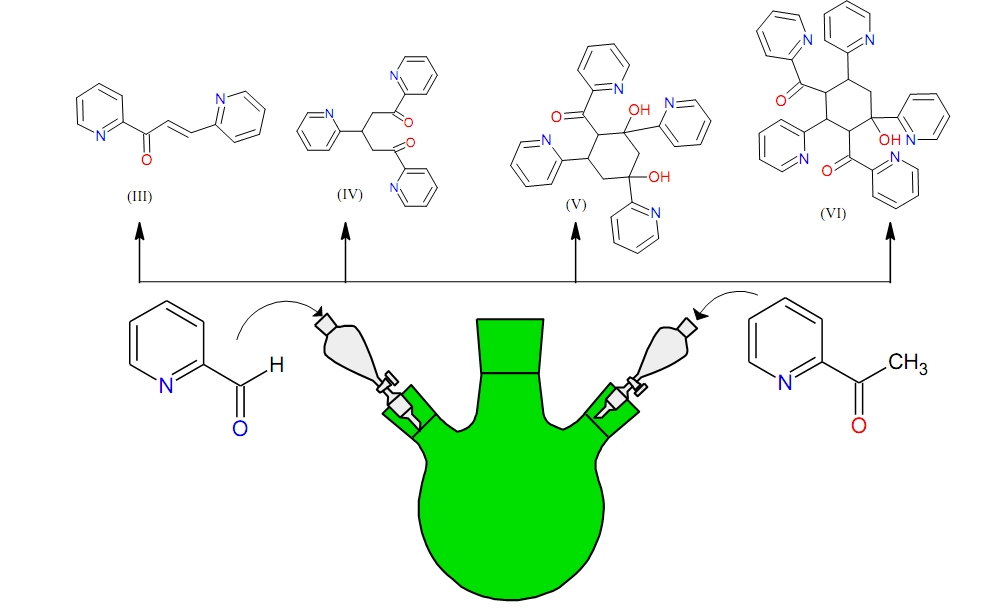
Graphical Abstract: The research is devoted to the study of unexpected products that formed as a result of the condensation reaction between 2-acetylpyridine and 2-formylpyridine under the Claisen-Schmidt reaction conditions. As a result, a sequence of reactions leading to the following compounds has been proposed: 1,3-bis (pyridin-2-yl) prop-2-en-1-one (3); 1,3,5-tri (pyridin-2-yl) pentane-1,5-dione (4); (2,4-dihydroxy-2,4,6-tri(pyridin-2-yl)cyclohexyl)(pyridin-2-yl)methanone (5) and (4-hydroxy-2,4,6-tri(pyridin-2-yl)cyclohexane-1,3-diyl)bis(pyridin-2-ylmethanone) (6) as well as 2-formylpyridine (1) and 2-acetylpyridine (2). The plausible mechanism of these chemical transformations has been proposed. All the obtained compounds demonstrate moderate antimicrobial, antifungal and antioxidant activity.

Downloads: 143






